AliveCor KardiaMobile ECG 6 Lead
AliveCor KardiaMobile ECG 6 Lead
SKU:ALI006
Couldn't load pickup availability
ecgThe latest KardiaMobile 6L from AliveCor produces a medical grade 6 lead ECG recording that gives you and your Doctor a more complete view of your heart using a free iOS and Android App. It's the first and only FDA cleared and CE-marked six lead personal ECG.
EKG on the go
-
Enjoy the peace of mind - KardiaMobile 6L lets you take an EKG anytime, anywhere. If you feel a symptom, or just want the peace of mind, simply take an EKG, and in 30 seconds know if your heart rhythm is normal or if Atrial Fibrillation is detected.
-
Trusted by doctors - Kardia has delivered more than 40 million EKGs and is recommended by leading cardiologists worldwide. With Kardia you can take EKGs between doctor visits, whenever you feel a symptom and email unlimited EKGs directly to your doctor.
- Using KardiaMobile 6L couldn't be easier - Simply open the Kardia app on your phone, and tap "record EKG. Place your thumbs on the top sensors, and touch the bottom sensor to the bare skin of your left leg (knee or ankle). In 30 seconds you'll see your results.
The KardiaMobile ECG has 2 electrodes in the front and one on the back. The two electrodes on the top are for your fingers, and the one on the bottom to contact the skin of your left leg. To record a 6 lead ECG hold the KardiaMobile 6L in your hands, placing your fingers on the front electrodes then touch the third electrode to the skin of your left knee or ankle. It's that easy.
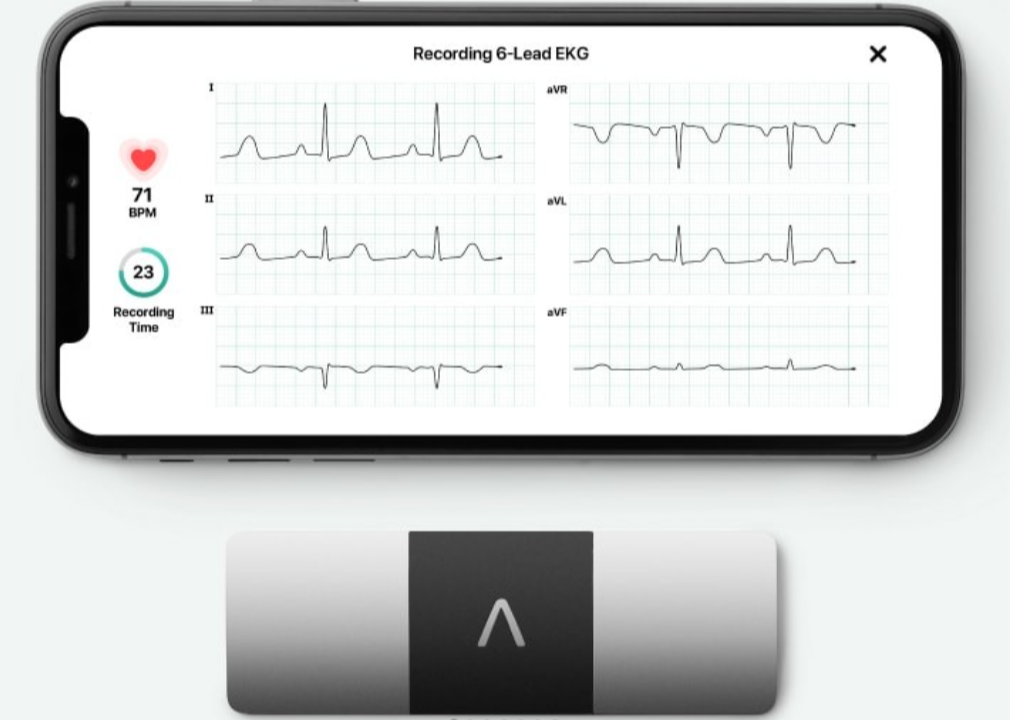
What six leads does it record? The KardiaMobile 6L displays and records 30 seconds of Lead I, Lead II, Lead III, aVR, aVL and aVF ECG in real time to your phone or tablet. This gives it the increased ability to identify cardiac arrhythmias such as atrial flutter, heart block and PVCs.
ECGs are stored in the app which at the end of the recording displays the ECG trace, heart rate and classifies the recording into 1 of the following categories.
- Atrial Fibrillation. The clinically validated algorithm will notify you to the presence of atrial fibrillation. If AF is detected, you will see the message "Possible AF Detected."
- Normal. If no rhythm abnormalities are detected, you will receive the message 'Normal'. This means that the heart rate is between 50 and 100 BPM, there are no or very few abnormal beats, and the shape, timing and duration of each beat is considered normal.
- Bradycardia a slow heart rate below 40-50 beats per minute.
- Tachycardia a fast heart rate above 100-140 beats per minute.
- Unclassified. The Normal Algorithm declares the ECG as Unclassified for ECGs that have a heart rate below 40 or above 140 bpm.
- Unreadable. 'Unreadable' implies that there was too much interference during the recording. This could be electrical or sound interference.
The free Kardia Basic App permits local storage of ECGs in the Kardia App. A user can view a complete history of the ECG's they have recorded on their device. Any stored ECG can also be printed or e-mailed as a PDF directly from the mobile device. ECG storage can be turned off within the App.
The App is available in the Apple App Store or via Google Play in the UK and Ireland. In a recent medtech innovation briefing (MIB) published by the National Institute for Health and Care Excellence (NICE) the KardiaMobile by AliveCor was positively reviewed and recognised.
Features include
- Record a medical grade 6 lead or 1 lead ECG
- Records Lead I, II, III, aVR, aVL and aVFL ECG in realtime
- Connects using Bluetooth Low Energy Technology
- 1 metre wireless range
- Uses 3 stainless steel electrodes
- No messy gels or wires
- Dimensions - 9.0 cm x 3.0 cm x 0.72 cm
- Weight - 24 grams
- Approximately 12 months (200 hours) operating time on one CR2016 battery
- App features automatic AF, normal ECG, Bradycardia, Tachycardia and interference detectors and voice recording
- Save and email a PDF or print an ECG from your device
- Professional, CE and FDA approved clinically validated medical device
Supplied with
- One 3 volt CR2016 coin cell battery
- Getting started guide
- Two year warranty
- UK based after sales support
- Manufacturers part number AC-019-NUA-A
Compatible Devices (Updated As Of 6/4/19)</>
Share